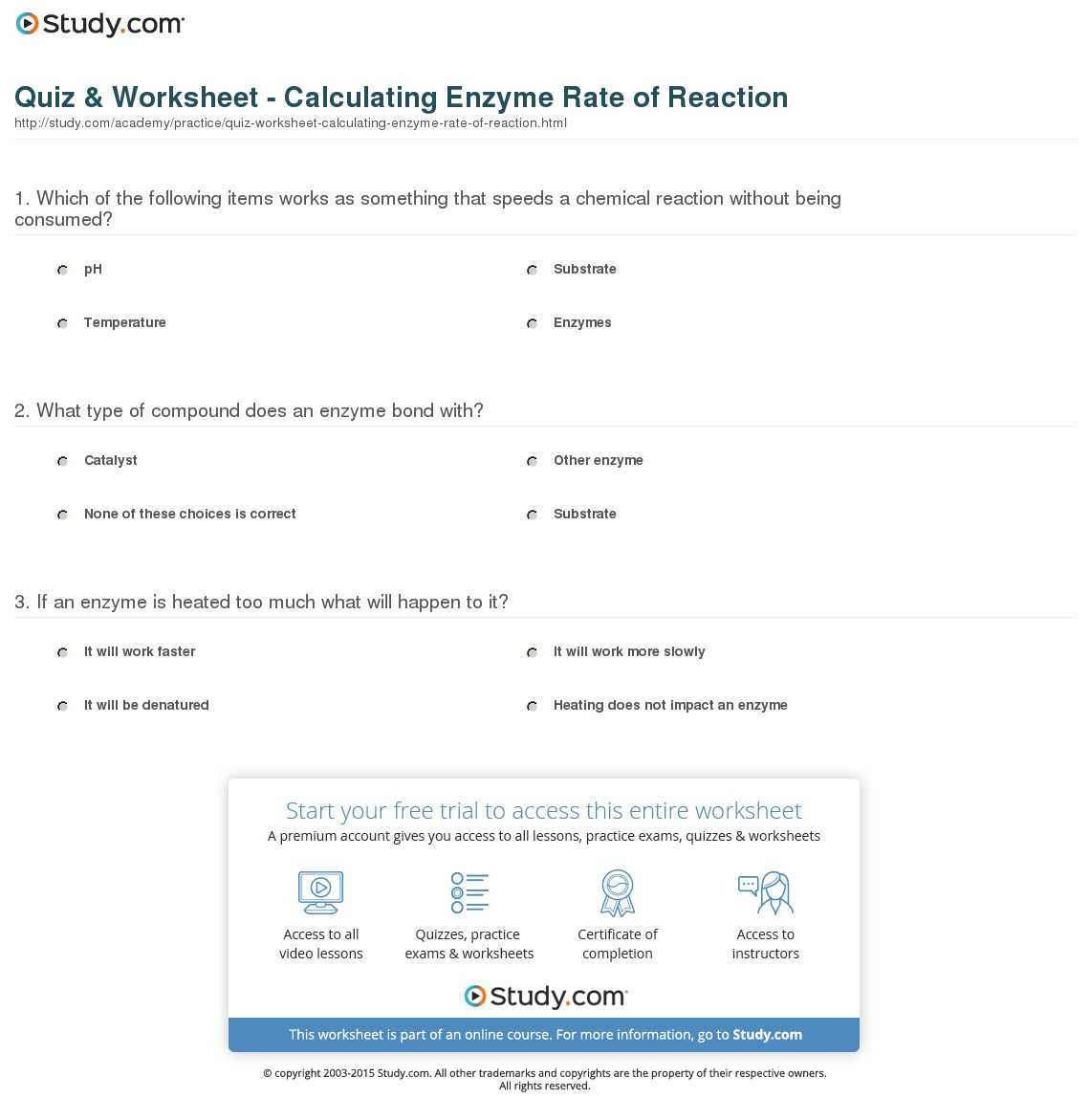
Reaction Rates Worksheet
Reaction Rates Worksheet - Use this reaction for the questions below: Web calculating rates of reaction. Web a student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. It includes a range of questions on concentration and a set of graph tasks. Web a revision homework or class worksheet with answers that covers rates of reaction in c6 gcse chemistry. Ii higher the concentration, the faster the reaction. A study of reaction _____ is called chemical _____. It is measured in terms of the _____ of the reactants. Still another factor which can change a reaction’s rate is a catalyst. Pearson education australia (a division of pearson australia group pty ltd) 2008.
Explain this observation, including a relevant equation in your answer. Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. Web the rate of a chemical reaction is a measure of how fast a reaction takes place. How will the rate change if the concentration of a is tripled? If a reaction is to occur, reacting particles must first _____ and this _____ must be effective. It is measured in terms of the _____ of the reactants. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid.
What happens to the concentrations of: Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of reaction, reaction rates and chemical equilibrium, sample exercise calculating an average rate of reaction. Web chemical kinetics is the study of the rates of chemical reactions; C6h12o6 & o2 as the reaction proceeds ? Ii higher the temperature, the faster the reaction.
H2o + co2 as the reaction proceeds ? Still another factor which can change a reaction’s rate is a catalyst. Ii higher the concentration, the faster the reaction. How will the rate change if the concentration of a is halved? Ii higher the concentration, the faster the reaction. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of reaction, reaction rates and chemical equilibrium, sample exercise calculating an average rate of reaction.
This is a substance which changes the rate of a reaction but remains, itself, un changed. 18 mean rate of reaction = quantity of product formed time taken mean rate of reaction = 30 15 Web the rate of a chemical reaction is a measure of how fast a reaction takes place. Still another factor which can change a reaction’s rate is a catalyst. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate.
Topics include collision theory, particles, factors that affect rate and reaction profiles/energy level diagrams with a variety of questions and challenging calculations. Web chemical kinetics is the study of the rates of chemical reactions; Reaction rate the speed at which a chemical reaction proceeds. E to change the concentration / keep the total volume the same.
Web A Student Investigated The Rate Of Reaction Between Calcium Carbonate (Marble Chips) And Hydrochloric Acid.
Designed as an introduction only:this product is designed to be used as an introd. D i b + d + e. How will the rate change if the concentration of a is tripled? A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid.
Web Chemical Kinetics Is The Study Of The Rates Of Chemical Reactions;
According to the collision theory, what 3 circumstances are needed for c6h12o6 & o2 to react? A study of reaction _____ is called chemical _____. How will the rate change if the concentration of a is halved? Example questions from the worksheet:
It Includes A Range Of Questions On Concentration And A Set Of Graph Tasks.
A reaction has the experimental rate law, rate = k[a]2. Provide definitions for the following terms; Worksheets and lesson ideas to challenge students to think hard about rates of reaction, graphs and collision theory (gcse and key stage 3) rates of reaction is a rich topic area for practicals that students really enjoy. B i a + c.
The Student Had Collected 30 Cm3 Of Gas Produced After 15 Seconds.
Calculate the mean rate of reaction from 0 to 15 seconds. Rates of reaction, collision theory, effect of variables on reaction rates including temperature, concentration, pressure, surface area, catalysts, and inhibitors.note: Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. Explain this observation, including a relevant equation in your answer.