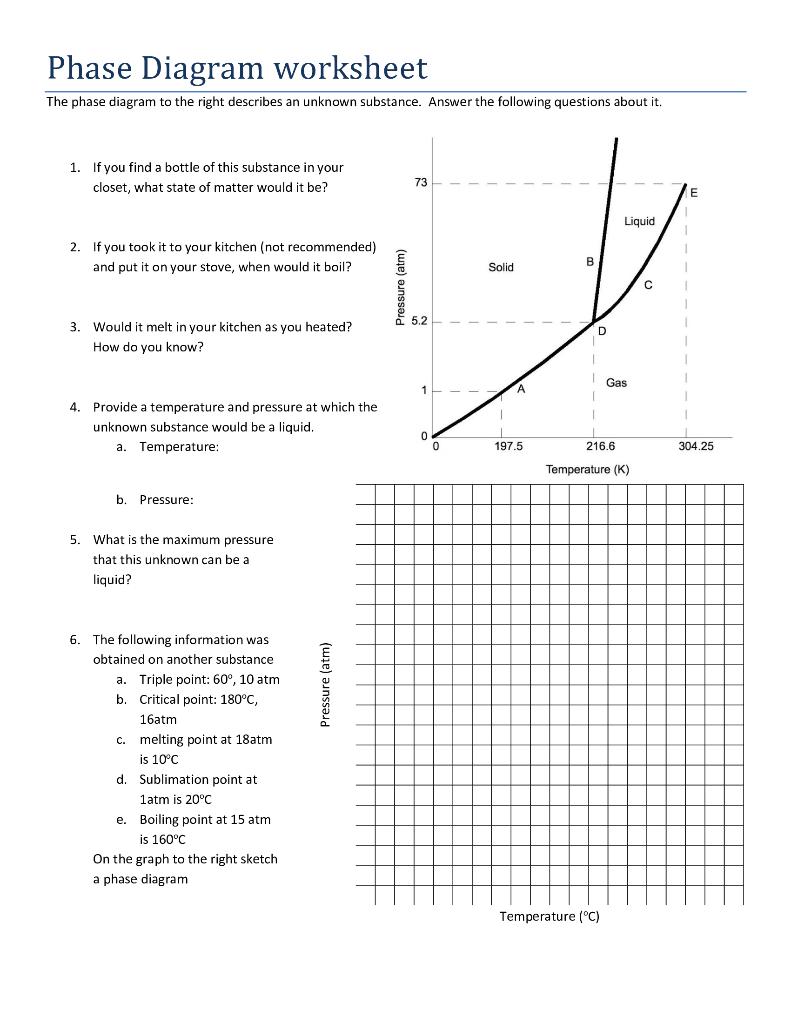
Phase Diagram Worksheet Answers
Phase Diagram Worksheet Answers - We can see this on the phase diagram because the point with the p=0.50 atm and t=100 k falls in the solid phase region. Which section represents the solid phase? Web phase change worksheet key. At point a, the beginning of observations, the substance exists in a solid state. Material in this phase has definite volume and shape. On the phase diagram, label the gas and liquid regions. A phase diagram is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases occur and coexist at equilibrium (at topic later on in the course). At point a, the beginning of observations, the substance exists in a solid state. Web answer the questions below in relation to the following generic phase diagram. Phase diagram worksheet #2 name________________ period_____ date______at standard temperature and pressure, bromine (br2) is a red liquid.
Use the graph and the words in the word bank to complete the statement. What section represents the liquid phase? At point a, the beginning of observations, the substance exists in a solid state. Material in this phase has definite volume and shape. What is the normal boiling point at 1 atmosphere of pressure? Which phase has the lowest free energy at 1.75 atm and 350 k? Web elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram:
At a pressure of 1 atmosphere, what is the normal freezing point? The slope of the lines separating the regions, which provides information about the relationship between temperature and pressure during phase changes. What section represents the liquid phase? Which phase has the lowest free energy at 1.75 atm and 350 k? Which section represents the solid phase?
Both phases exist on these lines: Answer the questions below in relation to the following generic phase diagram. Which phase has the lowest free energy at 1.75 atm and 350 k? A phase diagram is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases occur and coexist at equilibrium (at topic later on in the course). The curves indicate the conditions of temperature and pressure under which “equilibrium” between different phases of a substance can exist. Web explain the construction and use of a typical phase diagram;
We can see this on the phase diagram because the point with the p=0.50 atm and t=100 k falls in the solid phase region. The graph was drawn from data collected as a substance was heated at a constant rate. Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties; Web use the water phase diagram on the right to answer the following questions. The temperature and pressure values at specific points on the diagram, such as the melting and boiling points.
A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance: The graph was drawn from data collected as a substance was heated at a constant rate. Web phase diagram worksheet name: What letter represents the triple point?
Both Phases Exist On These Lines:
Both phases exist on these lines: Web this phase diagram worksheet key provides the answers and explanations for a worksheet about phase diagrams. Use this key to check your answers and learn more about phase diagrams. What letter represents the triple point?
We Can See This On The Phase Diagram Because The Point With The P=0.50 Atm And T=100 K Falls In The Solid Phase Region.
The phase diagram for bromine is shownbelow. Web elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: (3) see answer to 1. What section represents the gas phase?
Graphite Is The Most Stable Phase Of Carbon At Normal Conditions.
It helps students understand the different phases of matter and how they change under different conditions. This includes both constant pressure and constant temperature changes. Web 11.4 phase change diagrams. Use the graph to answer the following questions.
What Section Represents The Gas Phase?
Describe the supercritical fluid phase of. The graph was drawn from data collected as a substance was heated at a constant rate. Answer the questions below in relation to the following generic phase diagram. In your own words, what is the definition of a triple point?