Isotopes Worksheet Answers
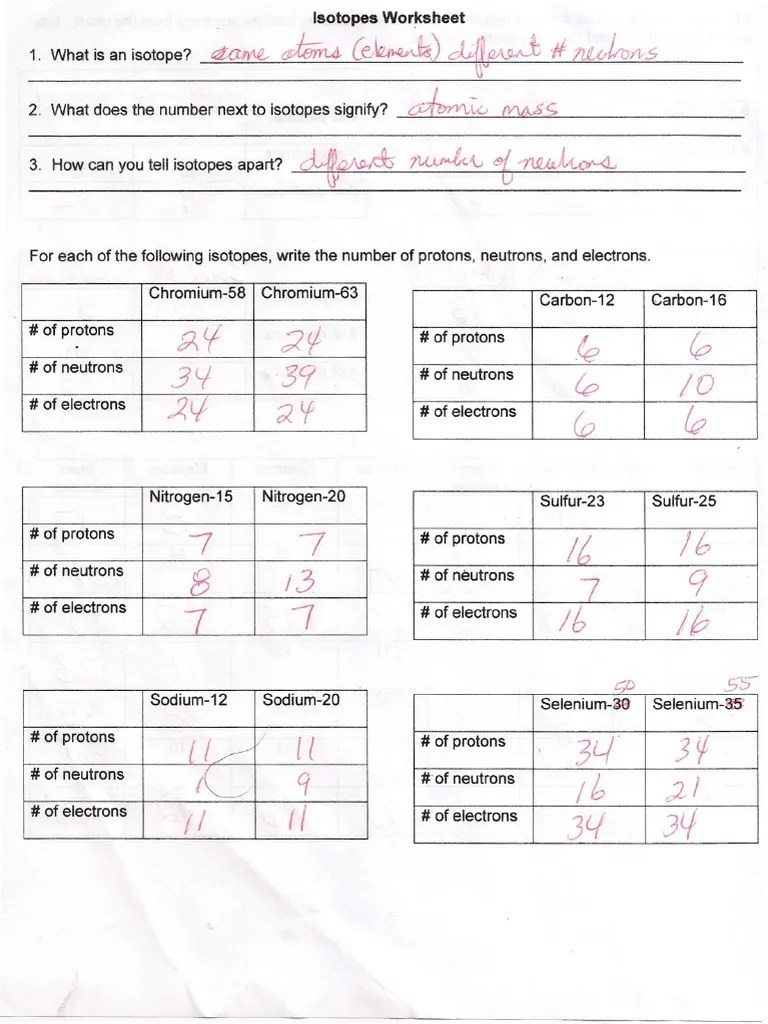
Isotopes Worksheet Answers - Web for each isotope shown, give the number of protons, neutrons, and electrons. What does the number next to isotopes signify? If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: The average mass of the balls is given by: Web atoms of the same element with different numbers of neutrons are called isotopes. How many protons and neutrons are in the second isotope? Give a reason for your answer. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). What does the number next to isotopes signify? Web the relative atomic mass (ar) of atoms is the average mass of all the different isotopes of an element (taking into account the amount of each isotope) on a scale where 12c atoms have a mass of exactly 12.
For each of the following isotopes, write the number of protons, neutrons, and electrons. Imagine you have 90 balls with mass 200 g, and 10 balls with mass 300 g. How can you tell one isotope from another? Explain, in terms of subatomic particles, what is meant by the term isotopes. _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. The numbers 12, 13, and 14 refer to the mass number d. What does the number next to isotopes signify?
For each of the following isotopes, write the number of protons, neutrons, and electrons. How many protons and neutrons are in the first isotope? Web in a sample of e there are two isotopes. How can you tell one isotope from another? Web fill in the blanks with the correct answers for the following:
Web isotopes of an element have the same atomic number (z) but have different mass numbers (a), because they have different numbers of neutrons. 19 protons, 22 neutrons, 19 electrons. _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. Web in a sample of e there are two isotopes. Web pdf, 1.36 mb. Students shared 1007 documents in this course.
The number 6 refers to the atomic number. A short worksheet to introduce or revise isotopes. \textcolor {f21cc2} {\text {number of neutrons}=\text {mass number. _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. How can you tell isotopes apart?
Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions. These isotopes are neutral (charge = 0). Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Exshare answers from the class, ensuring that all students are able to press 18o/16o in terms of voltage and resistance before allowing them to move on to calculate 18o/16o ratio from the data provided.
Web Pdf, 1.36 Mb.
_____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. Which isotope of lead is likely to be the most abundant? Isobars are nuclides of different elements (different \(z\)) with the same mass number (\(a\)). So different isotopes have different mass numbers but the same proton number.
How Many Protons And Neutrons Are In The First Isotope?
12c 6 13c 6 14c 6. Here are three isotopes of an element: The numbers 12, 13, and 14 refer to the ________________________ d. Consider the following illustrations in which protons are shown as pink spheres and neutrons are shown as green spheres.
Calculate The Average Atomic Mass Of Chlorine If Its Isotopes And % Abundances Are As Follows.
How can you tell isotopes apart? It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the qr code. 19 protons, 22 neutrons, 19 electrons. Web fill in the blanks with the correct answers for the following:
These Isotopes Are Neutral (Charge = 0).
6 protons & 6 neutrons e. Full written answers and a video explanation for this worksheet is also available. A short worksheet to introduce or revise isotopes. Web atoms of the same element with different numbers of neutrons are called isotopes.