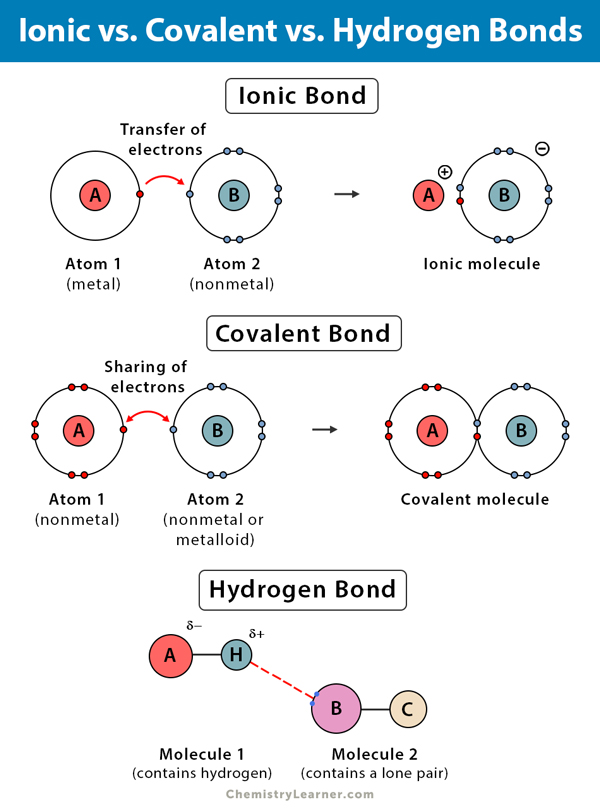
Does Hydrogen Form Ionic Bonds
Does Hydrogen Form Ionic Bonds - Such a bond is weaker than an. Web science & tech. Ionic bonds result from the attraction between. Web hydrogen ionic bonds are characterised by the hydrogen atom sharing an electron, forming neutral bonds, low bond energy indicating weak bonds, and acting as a poor. Web hydrogen bonds are one of the principal intermolecular forces. Hx− h x − forms ionic. Web hydrogen bonds are formed when a negative o, n, or f atom in one molecule attracts a positive h atom in another molecule. They are not ionic bonds,. Web as it turns out, the hydrogen is slightly negative. After bonding, the chlorine atom is now in contact with eight electrons in its outer shell, so it is.
Web hydrogen does form ionic bonds. Web hydrogen bonds are one of the principal intermolecular forces. Distinguish between nonpolar and polar covalent bonds. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Web hydrogen ionic bonds are characterised by the hydrogen atom sharing an electron, forming neutral bonds, low bond energy indicating weak bonds, and acting as a poor. Web as it turns out, the hydrogen is slightly negative. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons.
The oxygen atom of dmso form a hydrogen bond to the cation [emim] \(^+\). Identify the key difference between ionic and covalent bonds. Web hydrogen ionic bonds are characterised by the hydrogen atom sharing an electron, forming neutral bonds, low bond energy indicating weak bonds, and acting as a poor. Web hydrogen does form ionic bonds. Covalent and ionic bonds are intramolecular forces.
Identify the key difference between ionic and covalent bonds. Web atoms interact with each other through the formation of chemical bonds. Web as it turns out, the hydrogen is slightly negative. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. Web hydrogen ionic bonds are characterised by the hydrogen atom sharing an electron, forming neutral bonds, low bond energy indicating weak bonds, and acting as a poor. Hydrogen bonds are intermolecular forces;
Such a bond is weaker than an. Web distinguish between ions, cations, and anions. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Hx− h x − forms ionic. The oxygen atom of dmso form a hydrogen bond to the cation [emim] \(^+\).
This is because the oxygen atom, in addition to forming bonds. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. They are not ionic bonds,. Although not as bare hx+ h x +, it can form ionic bonds in the form of hx− h x − ( hydride anion ).
The Oxygen Atom Of Dmso Form A Hydrogen Bond To The Cation [Emim] \(^+\).
This is because the oxygen atom, in addition to forming bonds. Web the rdfs of the individual hydrogen bonds were averaged over all three ring protons. Electrovalency, electrovalent bond, heteropolar bond, polar bond. Web hydrogen ionic bonds are characterised by the hydrogen atom sharing an electron, forming neutral bonds, low bond energy indicating weak bonds, and acting as a poor.
Web Distinguish Between Ions, Cations, And Anions.
Such a bond is weaker than an. A special class are ionic hydrogen bonds (ihbs) that form between ions and molecules with bonds strengths of. Web science & tech. Distinguish between nonpolar and polar covalent bonds.
Identify The Key Difference Between Ionic And Covalent Bonds.
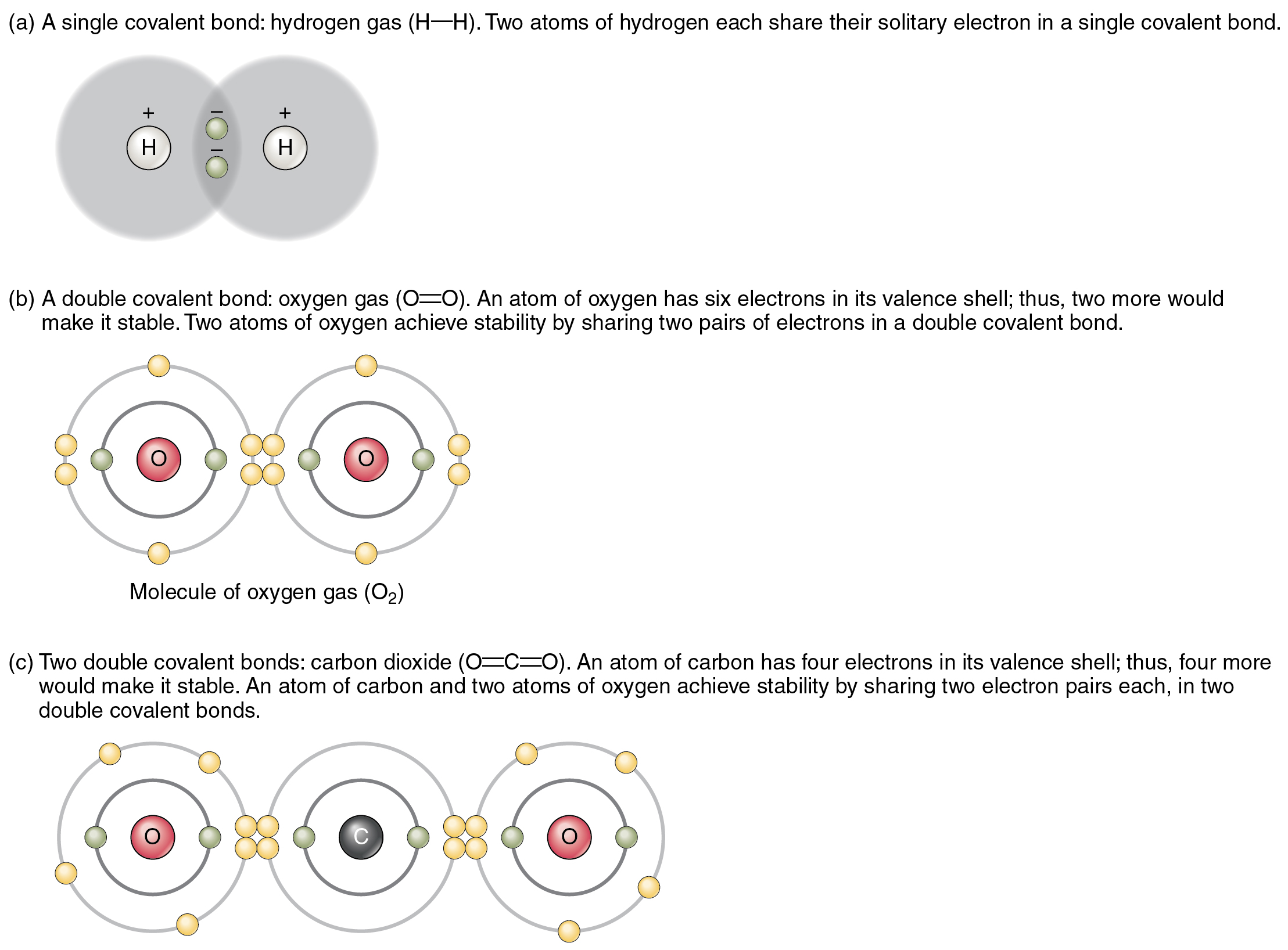
Web a hydrogen atom with one electron and a chlorine atom with 17 electrons. They are not ionic bonds,. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Covalent and ionic bonds are intramolecular forces.
Hx− H X − Forms Ionic.
Web hydrogen bonding is the strongest type of intermolecular bond. A bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Sometimes in compounds such as hcl there is an strong ionic bond but in compounds like ch4 hydrogen. I understand that it's a covalent bond, but to my uneducated mind isn't it possible to have the carbon give 1 electron to each of the hydrogens and form an ionic.


.PNG)


