Clausius Clapeyron Equation E Ample
Clausius Clapeyron Equation E Ample - 2) set up equation with values: T 2 = 325.95 k. Δh vap is the enthalpy of vaporization of the solution. Web the clausius clapeyron equation includes: Next, apply the clausius clapeyron equation and solve for p 2: Web where p1 and p2 are the vapor pressures at two temperatures t1 and t2. Web using the values r = 8.3145 joules per k and λ = 40.65 kilojoules per mole, the above equation gives t = 342 k (69 °c) for the boiling temperature of water, which is barely enough to make tea. \[\frac{d ln p}{dt} = \frac{\delta h_{vap}}{rt_{2}}\] \frac {dp} {dt} dt dp. P f p i = − δ vap h r ( 1 t f − 1 t i).
Next, apply the clausius clapeyron equation and solve for p 2: The clausius clapeyron equation predicts the rate at which vapour pressure increases per unit increase in temperature for a substance's vapour pressure (p) and temperature (t). \frac {dp} {dt} = \frac {h} {t \cdot \delta v} dt dp = t ⋅ δv h. Web the clapeyron equation can be developed further for phase equilibria involving the gas phase as one of the phases. Let's have a closer look at two vapor pressure equations: Web using the values r = 8.3145 joules per k and λ = 40.65 kilojoules per mole, the above equation gives t = 342 k (69 °c) for the boiling temperature of water, which is barely enough to make tea. We can further work our the integration and find the how the equilibrium vapor pressure changes with temperature:
In the case of vaporization, the change in molar volume can be expressed. This equation was suggested by b. Integration (with the assumption that δhfus/δv δ h f u s / δ v does not change much over the temperature range) yields. Web the clausius clapeyron equation includes: Web how to calculate vapor pressure?
At 100 o c the rate of increase of vapour pressure of steam is 27.1 mm hg per celsius degree, and a gram of steam occupies 1674 cm 3. In the case of vaporization, the change in molar volume can be expressed. Web the clausius clapeyron equation includes: R is the ideal gas constant =. T 2 = 325.95 k. Web t 1 = 287.85 k.
Integration (with the assumption that δhfus/δv δ h f u s / δ v does not change much over the temperature range) yields. Web how to calculate vapor pressure? P f p i = − δ vap h r ( 1 t f − 1 t i). Next, apply the clausius clapeyron equation and solve for p 2: — derivative of pressure with respect to.
\frac {dp} {dt} dt dp. 2) set up equation with values: Moreover, the application of van’t hoff to gas. Dp = δhfus δv dt t d p = δ h f u s δ v d t t.
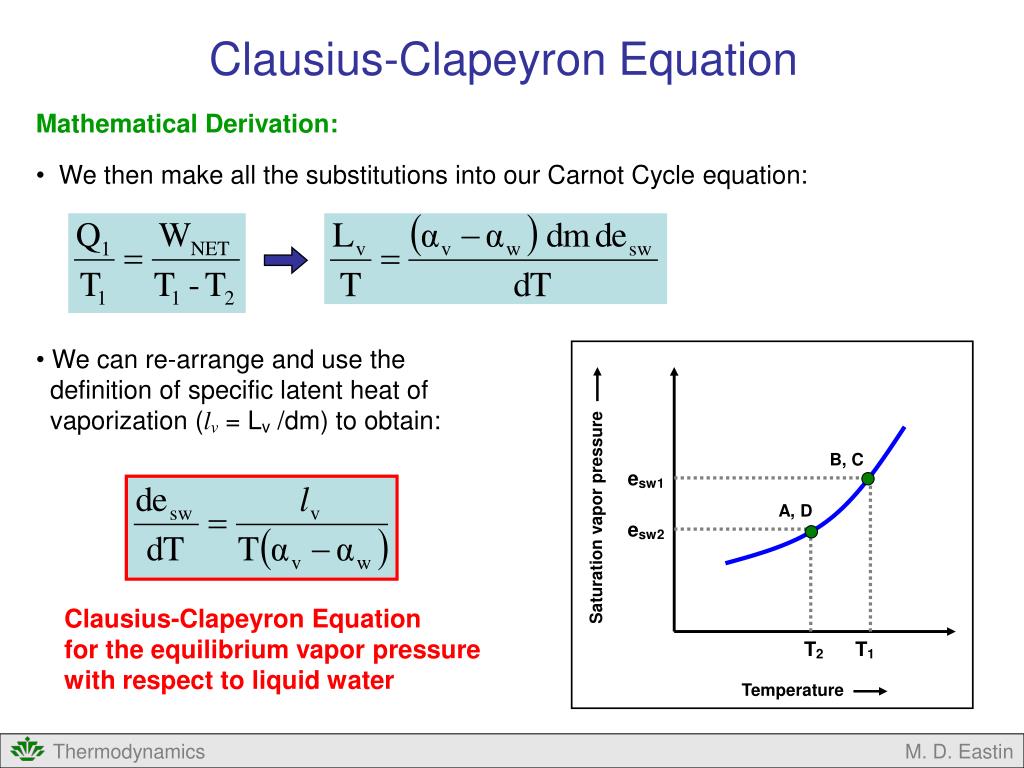
We Can Further Work Our The Integration And Find The How The Equilibrium Vapor Pressure Changes With Temperature:
It is often used to calculate vapor pressure of a liquid. R is the ideal gas constant =. Next, apply the clausius clapeyron equation and solve for p 2: Δh vap is the enthalpy of vaporization of the solution.
\Frac {Dp} {Dt} = \Frac {H} {T \Cdot \Delta V} Dt Dp = T ⋅ Δv H.
\[\frac{d ln p}{dt} = \frac{\delta h_{vap}}{rt_{2}}\] \frac {dp} {dt} dt dp. To do so, the heat of vaporization and the specific volumes must be known functions of temperature. (1) in the literature, this is often further approximated as a rate of 7%/°c [ panthou et al., 2014 ].
Web The Clausius Clapeyron Equation Derivation.
T 2 = 325.95 k. In the case of vaporization, the change in molar volume can be expressed. Dp = δhfus δv dt t d p = δ h f u s δ v d t t. Web to find the change in temperature, use the clapeyron equation (equation 8.4.4 8.4.4) and separating the variables.
At 100 O C The Rate Of Increase Of Vapour Pressure Of Steam Is 27.1 Mm Hg Per Celsius Degree, And A Gram Of Steam Occupies 1674 Cm 3.
T 2 = 52.8 °c + 273.15. — derivative of pressure with respect to. Moreover, the application of van’t hoff to gas. Web the clapeyron equation can be developed further for phase equilibria involving the gas phase as one of the phases.