Atomic Structure And Isotopes Worksheet Answers
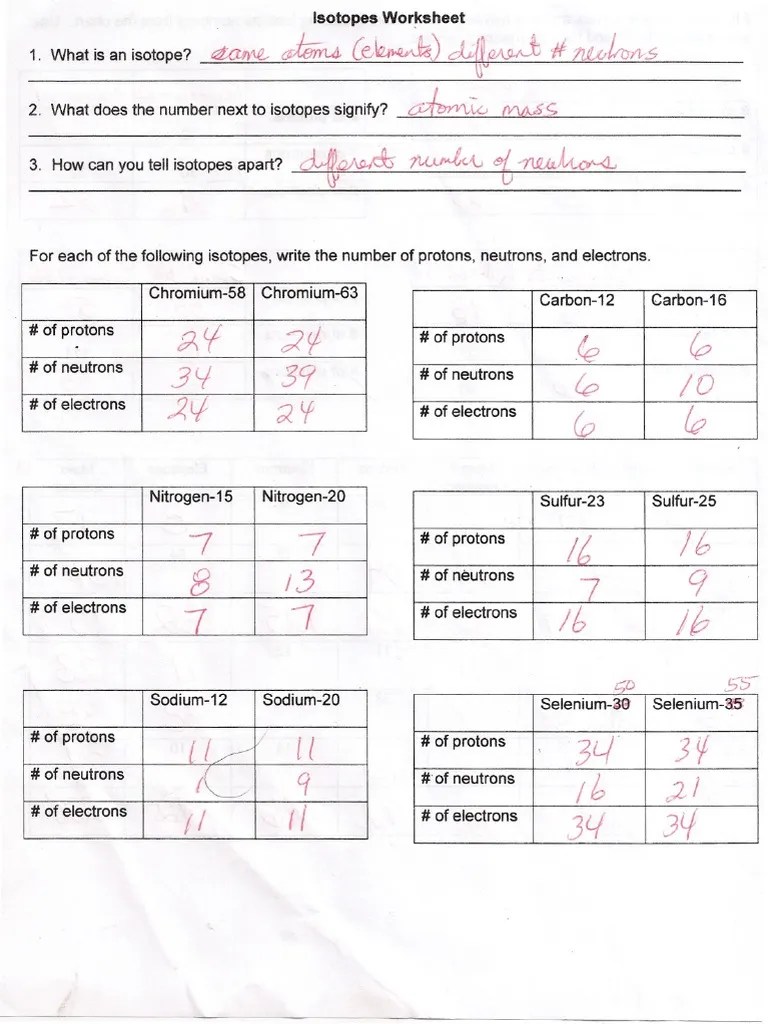
Atomic Structure And Isotopes Worksheet Answers - A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. 2 question is aimed at understanding the. Did this video help you? The lesson pack covers atoms, elements and isotopes. Calculate average atomic mass and isotopic abundance. (3) electron (1) neutron (1) proton (1) b) what is the atomic mass of this atom? Use of 87 × 6.9 and 88 × 82.9 and 10.2 anywhere. 81w 36 kr sld 37 kdu. Understand the relationship between the masses of isotopes and the atomic weight of an element; Proton p+ 1 +1 nucleus.
Protons and neutrons are in the nucleus. 2 question is aimed at understanding the. Web worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about atomic structure and isotopes (gcse and key stage 3) atomic structure teacher brief. Web atomic structure and the periodic table chapter 4 worksheet part a given the following isotopes, determine the atomic number, the mass number, the number of protons, electrons and neutrons. Atoms are the fundamental building blocks of matter and are built from protons, neutrons and electrons. Use of 87 × 6.9 and 88 × 82.9 and 10.2 anywhere. All substances are made of tiny particles of matter called atoms which are the building blocks of all matter.
The lesson pack covers atoms, elements and isotopes. Calculate average atomic mass and isotopic abundance. Never trust an atom, they make up everything! 2 question is aimed at understanding the. Click the card to flip 👆.
So different isotopes have different mass numbers but the same proton number. Never trust an atom, they make up everything! Understand the structure of atoms, isotopes, and ions; Or isotopes, taking into account the _____ of each isotope. Name symbol mass charge location. State the periodic law and explain the organization of elements in the periodic table.
All hydrogen atoms contain one proton (and one. Isotope symbo 131 il s3 1 ##### us |he. All substances are made of tiny particles of matter called atoms which are the building blocks of all matter. So different isotopes have different mass numbers but the same proton number. Recap the nuclear model of an atom.
A) the diagram shows an atom of beryllium. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Protons and neutrons are in the nucleus. Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion.
Never Trust An Atom, They Make Up Everything!
Define isotope, relative atomic mass (ram) calculate ram from isoptope data. Protons electrons neutrons isotope name Atoms are the fundamental building blocks of matter and are built from protons, neutrons and electrons. 3.1.7 oxidation, reduction and redox equations.
Web Türkiye Ortaokul Fen Bilimleri.
3.1.1.2 mass number and isotopes. Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Protons and neutrons are in the nucleus.
Web Atoms Of The Same Element May Have Different Numbers Of Neutrons.
(3) electron (1) neutron (1) proton (1) b) what is the atomic mass of this atom? Become familiar with the periodic table Define the unified atomic mass unit and average atomic mass. Electrons (in a neutral charge atom only!);
(1) Atomic Mass = 9 (1) C) Which Parts Make Up The Nucleus Of The Atom?
The total number of protons and neutrons in a nucleus. The number of subatomic particles in an atom can be. Understand the relationship between the masses of isotopes and the atomic weight of an element; Web atomic structure and the periodic table chapter 4 worksheet part a given the following isotopes, determine the atomic number, the mass number, the number of protons, electrons and neutrons.