Atomic Drawing Of O Ygen
Atomic Drawing Of O Ygen - Web 32k views 2 years ago. The electron shells are shown, moving outward from the nucleus. So that they’d have a bit of context, i went over the basic parts of an atom (protons, neutrons, and electrons) and made it clear that the name of. In the form of shells. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Oxygen is in group 6 of the periodic table. We’ll use a bohr diagram to visually represent where the electrons are. Therefore, an oxygen atom will have two electrons in the first shell and six in the 2nd shell. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web i started it off by having the students memorize the first 20 elements (h through ca), in their correct order — by atomic number — over their winter break.
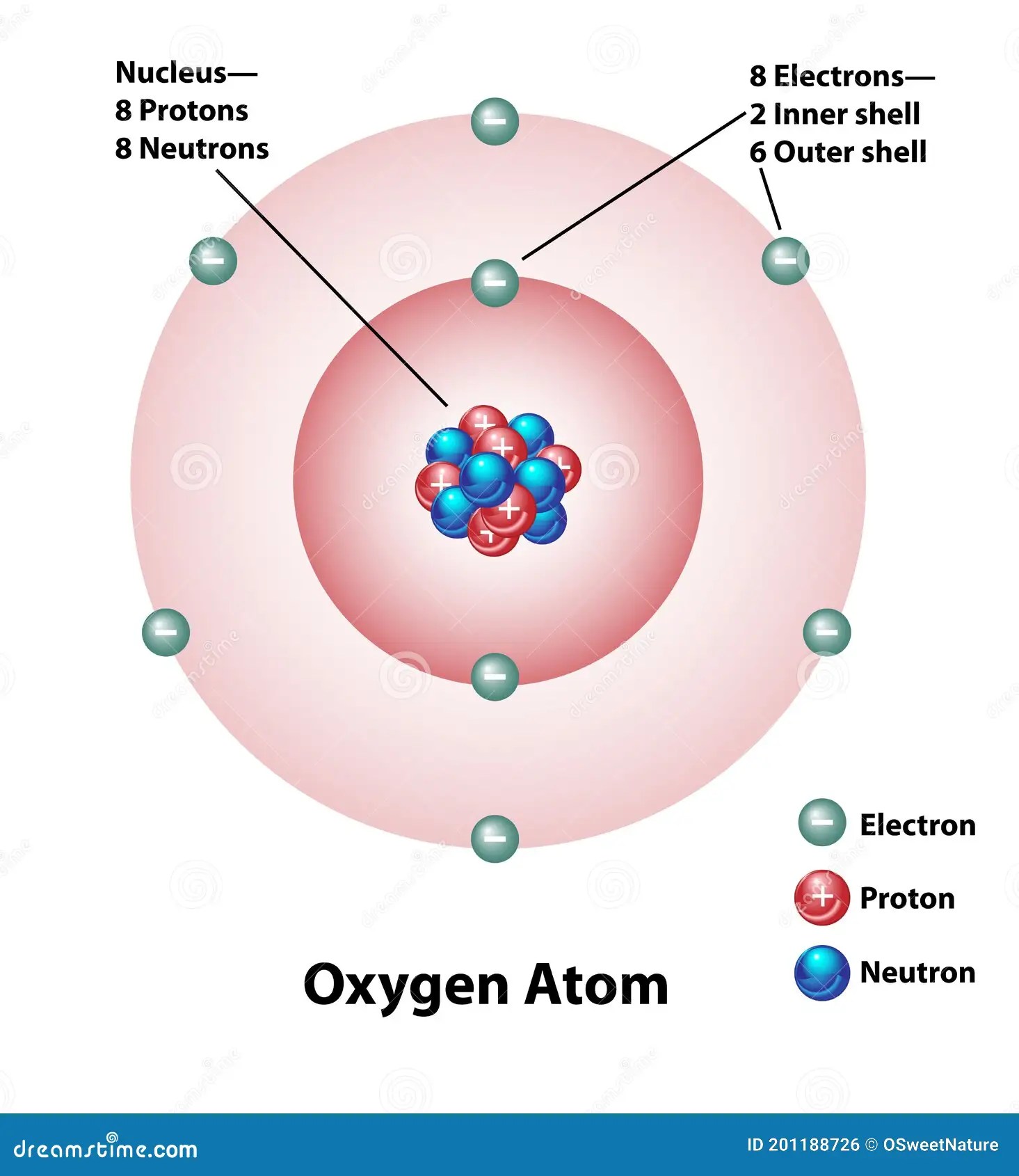
• the electronic configuration of oxygen is [he] 2s 2 2p 4. Oxygen has eight protons and eight neutrons in its nucleus, and eight electrons in two shells. The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the. The oxygen atom belongs to the 16 th group of the periodic table. In the form of shells. Web the atomic number of oxygen is 8. This simulation is part of the phet project, a leading provider of free online stem resources.
Oxygen is in group 6 of the periodic table. Web å 1 å = 100 pm = 10 − 10 m. This is a picture of an oxygen molecule. In this model, the black cloud represents the volume of space where electrons are likely to be found. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions.
A diagram of an oxygen atom. • the electronic configuration of oxygen is [he] 2s 2 2p 4. Web we recommend using the latest version of chrome, firefox, safari, or edge. 1 fm = 10 − 15 m. The video uses kr as an example, but the process is exactly as the same as what you need to do for oxygen. In the form of orbitals.
Two oxygen atoms will each share two electrons to form. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. The electronic structure of oxygen is: Web each element, when electrically neutral, has a number of electrons equal to its atomic number. 1 fm = 10 − 15 m.
It has an atomic weight of 15.999 and a mass number of 16. Let’s talk about orbitals first. For each electron shell atom diagram, the element symbol is listed in the nucleus. Two electrons will fill the first shell.the remaining 6 electrons will go into the second shell.
Sources, Facts, Uses, Scarcity (Sri), Podcasts, Alchemical Symbols, Videos And Images.
What is an oxygen molecule? Note that the 1s orbitals are significantly lower in energy than the 2s orbitals. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. It has an atomic weight of 15.999 and a mass number of 16.
• The Chemical Symbol Of Oxygen Is O.
126k views 12 years ago. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Consequently, the energies of the 2s and 2p orbitals of hydrogen are the same; For each electron shell atom diagram, the element symbol is listed in the nucleus.
Electrons Are Represented By Dots Or Crosses And Are Positioned In Energy Levels, Or ‘Shells’, Around The Central Nucleus.
Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Two covalent bonds and make an oxygen molecule ( o 2 ). Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. The video uses kr as an example, but the process is exactly as the same as what you need to do for oxygen.
In This Video We'll Look At The Atomic Structure And Bohr Model For The Oxygen Atom (O).
Therefore, the order of the number of electrons in each shell of the oxygen (o) atom is 2, 6. This simulation is part of the phet project, a leading provider of free online stem resources. The darker the region, the more likely electrons are to be found there. • the electronic configuration of oxygen is [he] 2s 2 2p 4.